
Many lung cancer patients now have access to a potentially life-saving medication.
Osimertinib, sold under the brand name Tagrisso, is available to patients with Stage 1B-3A lung cancer who have a certain genetic mutation and have had surgery to remove cancerous tumors.
Among those patients, Tagrisso was shown to reduce the five-year risk of recurrent cancer by up to 73% and the risk of death by up to 51%, according to research published in The New England Journal of Medicine over the summer.
SHOULD YOU BE SCREENED FOR LUNG CANCER BASED ON NEW GUIDELINES FOR HIGH-RISK PATIENTS?
“In the world of oncology, that is earth-shattering,” Dr. Faiz Y. Bhora, chief of thoracic surgery and central region chair of surgery at Hackensack Meridian Health in New Jersey, told Fox News Digital.
“In the past, medical oncologists were happy with 5% or 10% — and now we’re talking about in excess of 50% improvement in survival.”

Dr. Faiz Y. Bhora, chief of thoracic surgery and central region chair of surgery at Hackensack Meridian Health in New Jersey (left), treated Kim Mosko, 67 (right), who is taking Tagrisso after surgery to remove a cancerous tumor. (Hackensack Meridian Health)
Bhora, who has prescribed the medication to several of his lung cancer patients, spoke about the “groundbreaking” results he’s seen in his own practice.
“We’re truly in the era of personalized medicine,” Bhora also told Fox News Digital. “We now have a lot of targeted therapies that work well for patients who have mutations in their tumors.”
LUNG CANCER: TYPES, SYMPTOMS AND TREATMENT OPTIONS
For this particular medication, patients who have a genetic mutation called EGFRm — and who have already had surgery — are viable candidates for Tagrisso.
“The pill helps prevent recurrence once the tumor is removed with surgery for those with the genetic marker,” he said.
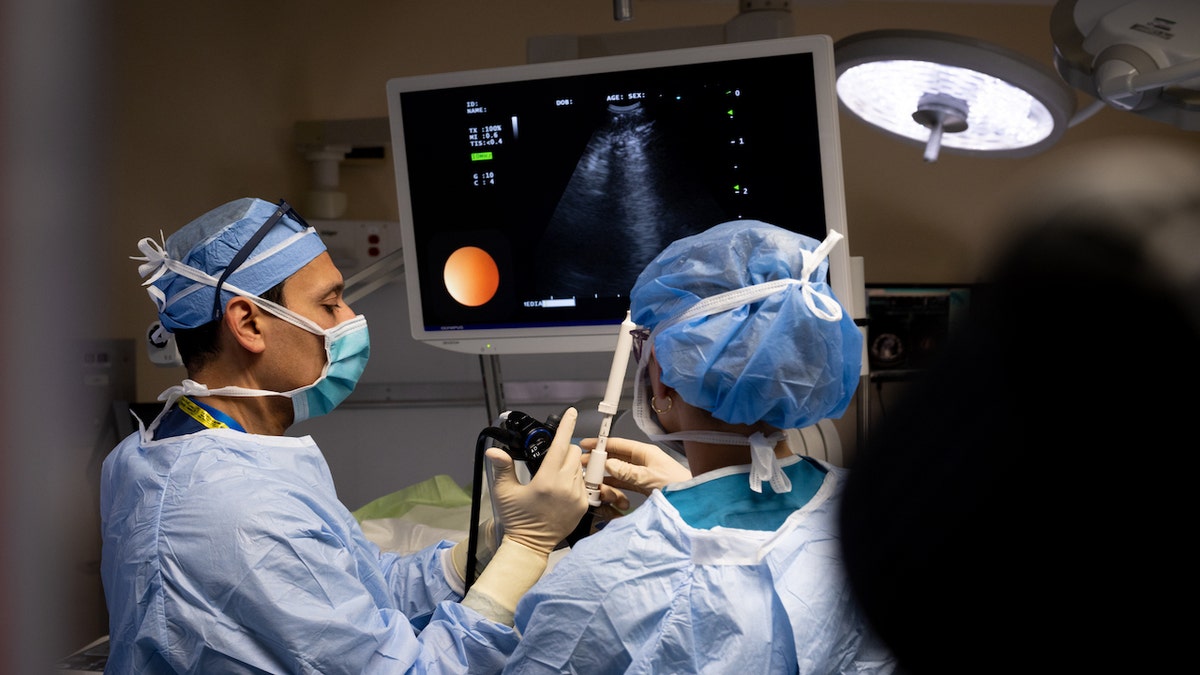
Dr. Faiz Y. Bhora (left) and his team prepare for surgery. “We have perhaps the most outstanding team of thoracic surgeons, who can perform the most complex operations robotically,” he told Fox News Digital. (Hackensack Meridian Health)
Patients with stage 4 lung cancer are also eligible for the pill if they have the EGFR mutation, even without having had surgery, the doctor noted.
To determine if a patient has the mutation, a tissue sample is extracted from the tumor and tested. Results are typically obtained within 10 to 14 days.
A blood test is also available, Bhora said, with those results available within five to 21 days.
“We used to think of lung cancer as just a smoker’s illness. Now, we know that over 30% of individuals who develop lung cancer have never smoked.”
“I would say about 25% of patients with lung cancer end up having an ETFR mutation,” the doctor estimated.
Around 238,000 new cases of lung cancer are expected in 2023, according to the American Cancer Society (ACS), along with some 127,000 deaths.
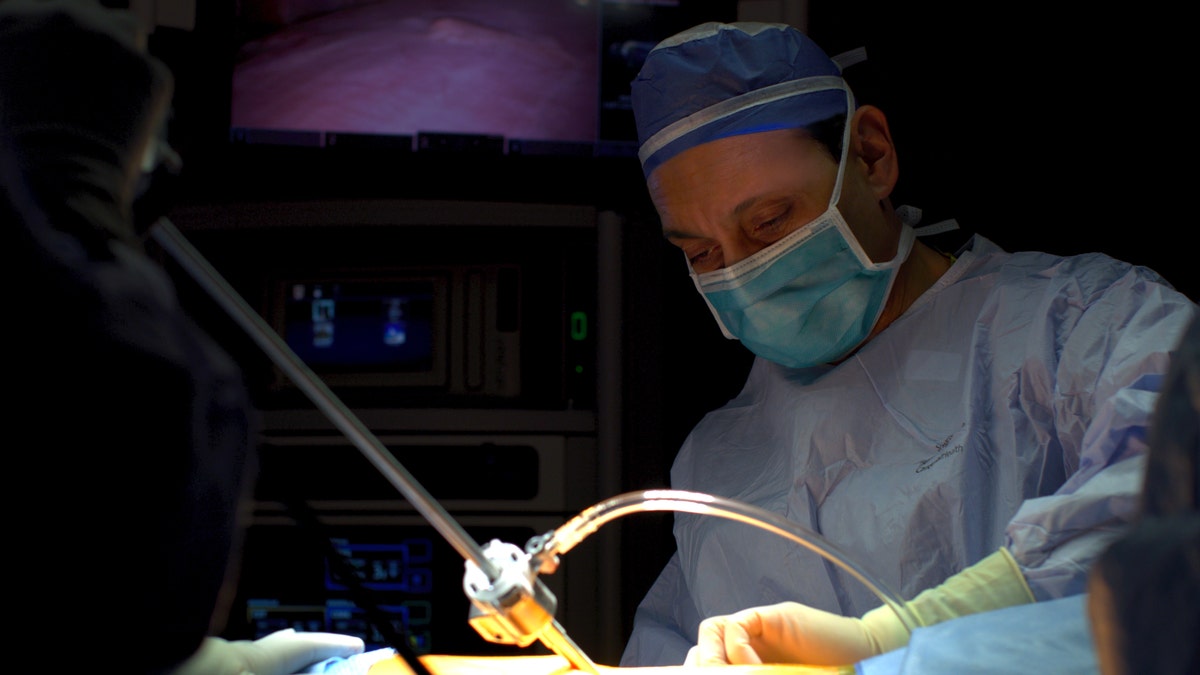
Dr. Faiz Y. Bhora is pictured during surgery. “Even with some stage 4 patients, we try to treat with systemic therapy first, and if there is residual disease we go in surgically to remove what’s left,” he said. (Hackensack Meridian Health)
“We used to think of lung cancer as just a smoker’s illness,” Bhora said. “Now, we know that over 30% of individuals who develop lung cancer have never smoked, and a lot of them are women.”
Looking ahead, he hopes to be able to prescribe the pill soon to patients upfront, before any other procedures or therapies, with the goal of shrinking tumors prior to surgery.
A patient’s story
Kim Mosko, 67, was diagnosed with stage 2A lung cancer in February 2023.
The mother of two had robotic surgery at Hackensack Meridian just a couple of weeks later.

Kim Mosko, 67, has been taking Tagrisso for 3½ months. “Overall, my experience has been positive, and I am grateful that this medication is available,” she said. (Hackensack Meridian Health)
“The lobectomy was performed by robotic surgery and was an incredible experience,” she told Fox News Digital. “I owe all the doctors I see and consulted with a debt I can never repay.”
Next, she had four rounds of chemotherapy, which she completed at the end of June 2023.
“I absolutely believe that this medication is going to make sure the lung cancer will not return. I am planning on living for many more years.”
When Mosko’s doctors determined in July that she has the genetic mutation, they recommended that she take Tagrisso.
“I needed no persuasion at all,” she told Fox News Digital. “I will do whatever is necessary to treat this cancer and lengthen my lifespan.”

When Mosko’s doctors recommended that she take Tagrisso, “I needed no persuasion at all,” she told Fox News Digital. “I will do whatever is necessary to treat this cancer and lengthen my lifespan.” (Hackensack Meridian Health)
Mosko has now been taking Tagrisso for 3½ months, with plans to continue taking it daily for three years.
Her insurance covers the cost of the medication.
“Overall, my experience has been positive, and I am grateful that this medication is available,” she said.
TWO NEW CANCER PILLS SHOW ‘UNPRECEDENTED’ RESULTS IN BOOSTING SURVIVAL RATES AND PREVENTING RECURRENCE
She has experienced some side effects, such as a skin rash, diarrhea and fatigue, “all of which are manageable,” Mosko said.
“I don’t need to have hope,” she added. “I absolutely believe that this medication is going to make sure the lung cancer will not return. I am planning on living for many more years.”
Where to start
For lung cancer patients interested in Tagrisso, Bhora recommends seeing an experienced team of physicians to get tested for the genetic mutation.
At Hackensack, Bhora said, “we consider every patient a candidate for a cure.”
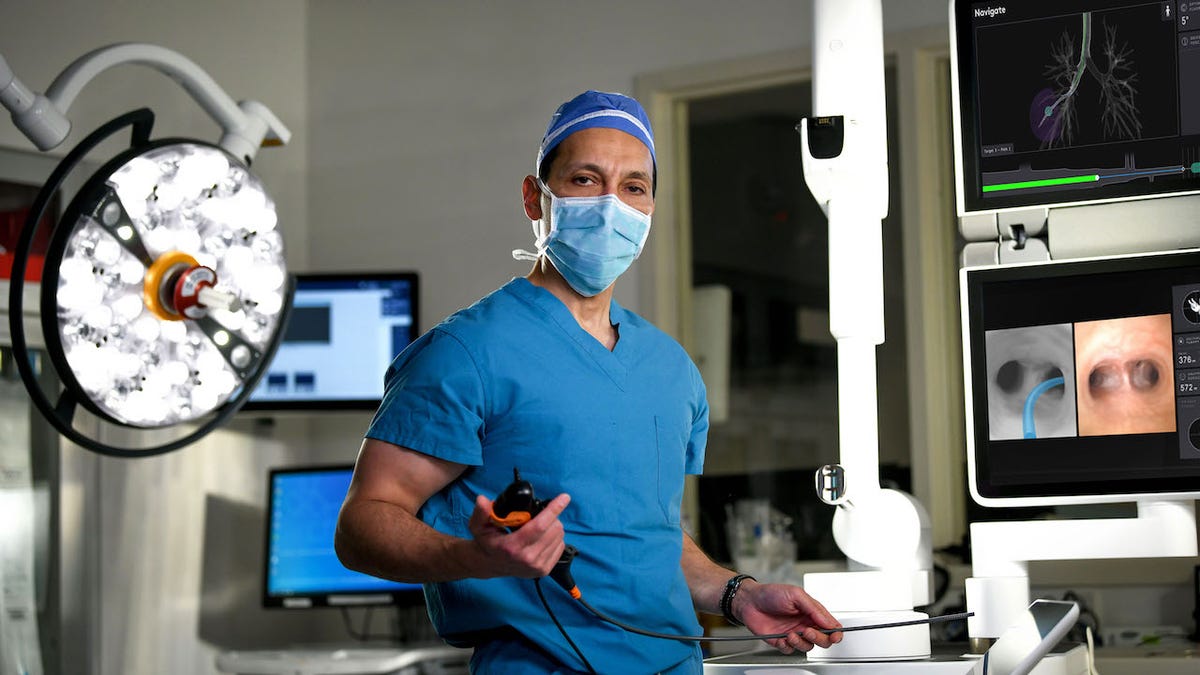
“This is a very optimistic time for patients who used to have what was universally considered a fatal disease,” Bhora said. (Hackensack Meridian Health)
With the advent of personalized therapies, he believes there is hope on the horizon for many lung cancer patients.
CLICK HERE TO SIGN UP FOR OUR HEALTH NEWSLETTER
“This is a very optimistic time for patients who used to have what was universally considered a fatal disease,” Bhora said.
“We are now in an era where in a vast majority of cases, we can either turn it into a chronic disease or aim for a complete cure.”
“We are now in an era where in a vast majority of cases, we can either turn it into a chronic disease or aim for a complete cure.”
Safety information
AstraZeneca, the maker of Tagrisso, indicates on its website that some side effects have been reported.

The logo for AstraZeneca is seen outside its North America headquarters in Wilmington, Delaware, March 22, 2021. AstraZeneca is the maker of Tagrisso. (REUTERS/Rachel Wisniewski/File Photo)
The most common of those include low white blood cell counts; low platelet counts; diarrhea; low red blood cell counts (anemia); rash; muscle, bone, or joint pain; mouth sores, fatigue, cough, dry skin; and changes in the fingernails, including redness, tenderness, pain, inflammation, brittleness, separation from the nailbed and shedding of the nail.
Although rare, some potentially serious side effects may affect the lungs, heart, eyes, skin, and blood and bone marrow.
Patients who experience bothersome or long-lasting side effects should consult with their health care provider, AstraZeneca states.
CLICK HERE TO GET THE FOX NEWS APP
They can also report any side effects to the U.S. Food & Drug Administration (FDA) by calling 1-800-FDA-1088.
For more Health articles, visit www.foxnews.com/health.

 Latest Breaking News Online News Portal
Latest Breaking News Online News Portal




